
The internet of medical things (IoMT) market is expected to grow significantly in the future, rising from $113bn in 2021 to reach $341.17bn by 2028, according to Global Informationa’s Global Digital Health Market Forecast (2021–2028).
The IoMT involves collecting and analysing data from internet-connected things, such as devices, equipment and facilities, and using it to glean new knowledge about patients’ conditions. Possible IoMT applications include direct management of patients’ conditions through measuring factors such as activity level, blood pressure and sleep. As well as improving their lives directly, using the IoMT can help doctors and hospitals work more efficiently and achieve better outcomes for patients. The IoMT is expected to help extend healthy life expectancy, reduce medical labour shortages, and improve the quality of medical and nursing care.
IoMT system configuration
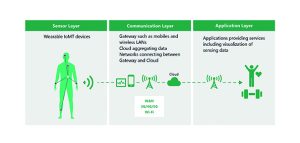
Figure 1: Three-layered IoMT services
An IoMT service is built from hardware, applications and networks, as shown in Figure 1.
The main components may include:
* Sensor layer (IoMT devices such as wearable sensors with network connectivity)
* Communication layer (gateways such as mobile devices and wireless LAN (IEEE 802.11x/Wi-Fi) routers; cloud aggregating data and networks (public network or internet) connecting between gateway and cloud
* Application layer (applications implemented in cloud or on mobiles providing services such as visualisation of sensing data).
Mobile phones are a good basis for IoMT services, principally because so many people own one. They are easy to connect to IoMT devices such as smartwatches using Wi-Fi and Bluetooth and can connect to the cloud via 4G (LTE) or 5G public networks.
Capturing data
From the IoMT device, sensing data is sent to mobiles or gateways via Wi-Fi or Bluetooth. Some IoMT devices have built-in SIM cards and can connect directly to public networks. Acquired data such as respiration rate, body temperature, pulse rate and blood pressure are basic information called vital signs. Blood oxygen saturation (SpO2) is sometimes included among these.
One of the major advantages of biometric data sensing by healthcare devices is that it is minimally invasive, requiring no blood sampling or body implant. A basic technology is photoplethysmography, which optically detects changes in blood vessel volume. An LED on the back of the smartwatch emits light (mainly green) at blood vessels in the wrist, with reflected light received by a photodetector. The smartwatch uses signal processing to extract regular fluctuations from the various noise components to obtain the pulse rate. When green and red LEDs are used together, transcutaneous arterial SpO2 can be estimated from the degree of haemoglobin binding.
The respiration rate can be estimated from the pulse rate using the body’s respiratory sinus arrhythmia, in which the pulse rate increases slightly during inhaling and decreases slightly during exhaling. Blood pressure is estimated from bloodflow based on pulse rate. In addition, sleep status is determined from body movements detected by the smartwatch accelerometer.
Hardware challenges
There are many challenges in achieving successful IoMT applications, principally connected to services, hardware and communications.
In terms of services, managing the measurement accuracy of acquired physical data and ensuring the security of cloud-aggregated personal data are major considerations. Other challenges are compliance with national and regional radio laws, and regulations and obtaining certification.
IoMT devices can connect wirelessly to mobiles and IoT gateways, and maintaining this communication connectivity is critical if they are to collect accurate medical data. Devices such as smartwatches and smart shoes use Bluetooth for easy pairing with mobiles and other devices.

Figure 2:
Electromagnetic noise in the system can interfere with and affect other circuits
For low power consumption, that is, in the 2.4GHz frequency band, Bluetooth is assigned as a licence-free, low-power radio station. Wi-Fi (IEEE 802.11x) is implemented for IoMT devices in stationary locations, such as smart scales, beds and other going-to-bed/getting-up sensors, and surveillance cameras.
With hardware, one of the key challenges for IoMT devices is downsizing. For example, a smartwatch, which is similar in size to a standard wristwatch, must contain a battery, charging circuit, microcontroller, communication function, display and other components, as well as various sensors. Limited battery power dictates the use of a design with low power consumption. Other important components include an analogue front-end to amplify weak electrical signals output by photodetectors and accelerometers, and a filtering process to separate noise components from the required information.
HF noise testing
Commercialisation of IoMT devices requires compliance with various test standards for high frequency (HF) noise. An emission test is used to verify that HF electromagnetic field noise from the IoMT device does not affect other equipment, along with an immunity (disturbance) test to verify that the IoMT device is not itself affected by such noise. Electromagnetic field noise emitted from the electronic circuit can interfere with, and distort, the weak signals from built-in sensors and can cause Wi-Fi and Bluetooth communication errors.

A typical source of electromagnetic noise in IoMT devices is switching power supplies (DC/DC converters) that generate harmonic noise. Clocks for microcomputers and memory are also noise sources.
Noise counter measures include physical separation of electronic circuits and antennas, adding EMI filters, configuration of board layouts and layers, and using physical shields.
Applicable tests for consumer IoMT devices are CISPR 32 Class B as defined by the International Special Committee on Radio Interference (CISPR) for emissions testing and CISPR 35 for immunity testing. In addition, IoMT devices with Wi-Fi and Bluetooth must comply with each national legislation on regulating radio use.
Security and personal data
Cyber security measures are another challenge. For example, in March 2023, the US Federal Food and Drug Administration issued new guidelines for medical device vendors. When developing new medical devices, the guidelines recommend or mandate designs that take cyber security into account, creation of software bill of materials and vulnerability assessment. Prompt provision of security updates throughout the product life is also expected, while non-compliant products are expected to lose their marketing authorisation.
Another issue is compliance with laws on protection of privacy information. Data collected by IoMT devices and analysed by cloud or mobile applications that can identify individuals could be classed as personal information and require special protection.
Future IoMT
Healthcare and medicine are converging in the IoMT market, where significant future growth is expected. IoMT services should help extend healthy life expectancy and improve quality of life, as well as encouraging people to work for as long as they can. Additionally, a healthier population reduces social security costs.
Telemedicine using IoMT is expected to become reality soon, offering medical services to many people, including those in remote areas, and developers will need to produce advanced IoMT devices and services to meet these expectations.
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News



