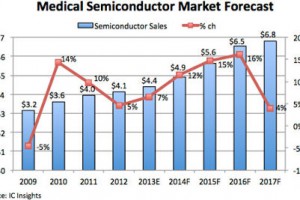
Medical electronics is expected to regain strength in the next three years after slowing since 2010 due to the weak global economy and efforts to curb healthcare costs in the U.S. and Europe, says IC Insights.
Medical Electronics
Content related to medical electronics
Pre-qualified batteries for medical and industrial devices
Staffordshire-based Accutronics has launched a range of pre-qualified Li-ion batteries for medical equipment and industrial electronics. Called VR420, “they are UL-recognised [UL2054], and tested to IEC62133 which is mandatory for medical equipment and becoming important in other markets”, technical marketing manager Neil Oliver told Electronics Weekly. To meet transportation regulations, stored energy is less than 100Wh and the products have ...
Know your MOPPS from your MOOPS in medical power supply design
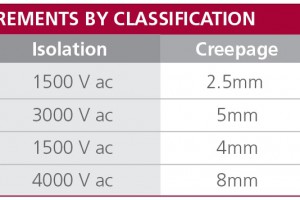
IEC/EN 60950 is a set of guidelines for the electrical safety of equipment connected to the public power grid, or mains. Now in its 3rd edition, IEC/EN 60601-1 is a further series of technical standards relating to the safety and performance of ‘Medical Electrical Devices’. The regulations are extensive and complicated but two things need to be appreciated when selecting ...
Haswell and its relevance to medical device design
When Intel introduced the 4th Generation of its Core i-Series earlier this year, the standout feature was the quad-core Core i7 4700EQ processor with long-term availability. It is particularly suitable for power-hungry applications in medical technology. Intel launches a new generation of processors every year. While the last processor generation introduced a new low-power 22-nm process technology, the novelty of the recently ...
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News