The voltage-flow curve is almost a straight line, and operation at 1MHz has been demonstrated.
“Precision control of how heat flows through materials has been a long-held but elusive dream for physicists and engineers,” said engineering professor Yongjie Hu (pictured). “This design principle takes a big leap toward that, as it manages the heat movement with the on-off switching of an electric field, just like how it has been done with electrical transistors for decades.”
It is a nano-scale sandwich device, built starting with an atomically-flat gold coating on a substrate.
A self-assembling mono-layer comes next, of ‘carboranethiol cage’ molecules (9-SH-o-C2B10H11(O9)).
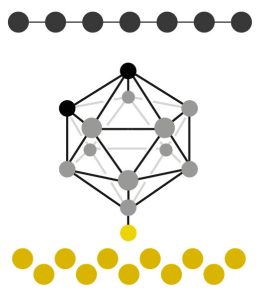 Physically, these molecules are multifaceted ball-like cages that stand up on the gold surface on a single stalk-like leg, attached via the sulphur atom.
Physically, these molecules are multifaceted ball-like cages that stand up on the gold surface on a single stalk-like leg, attached via the sulphur atom.
The carboranethiol cage – boron atoms are shown in grey, carbon atoms in black (graphene across the top), sulphur in yellow and gold is at the bottom
Across the top of this forest of cages is draped sheet of single-layer graphene, which is held ~1nm from the gold by the height of the cage molecules.
The controlling potential bias is applied between the gold surface and a top contact above the graphene and touching it.
Heat flows up from the gold surface towards the top electrode, through the carboranethiol molecules and graphene.
Conductivity of the sandwich varies from below 10MW/m2/K to above 130MW/m2/K depending on voltage bias between the gold and the electrode.
In operation (please forgive Electronics Weekly for the following hand-waving explanation…), electrons shared by the sulphur and gold atoms to form their covalent bond, shift under the influence of the applied electric control field, which weakens or strengthens the bond, consequently changing local thermal conductivity out of the gold surface. Something similar happens at the graphene interface, but based on Van der Waals attraction bonding rather than covalent bonding.
For a proper explanation of operation, the work is described in ‘Electrically gated molecular thermal switch‘, published in Science (abstract available without payment).
That formula
How do you read the formula 9-SH-o-C2B10H11(O9)?
“The first 9 and last 9 mean the position of the SH group attached to the C2B10H11 cage molecule”, Professor Hu told Electronics Weekly. “In addition, the ‘o’ stands for ‘ortho-‘, which is one type of the carborane cage molecule, indicating where the 2 carbon atoms are located.”
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News