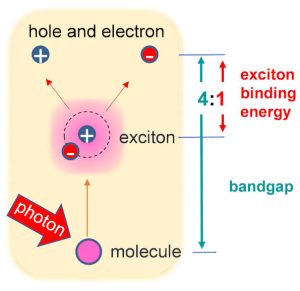
It is that the exciton binding energy in a material is a quarter of its transport bandgap, regardless of the material.
“A previously unpredicted nature of exciton binding energies in organic semiconductors was revealed,” said engineering professor Hiroyuki Yoshida. “Our study contributes to the understanding of the mechanism of excitons in organic semiconductors. Moreover, these concepts are not limited to organic semiconductors, but can also be applied to a wide range of molecular-based materials, such as bio-related materials.”
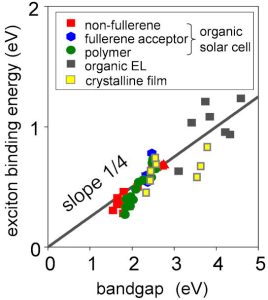 The team measured exciton binding energies for 42 organic semiconductors, including 32 solar cell materials, seven organic LED materials and three crystalline pentacene compounds.
The team measured exciton binding energies for 42 organic semiconductors, including 32 solar cell materials, seven organic LED materials and three crystalline pentacene compounds.
To compute the exciton binding energies, the researchers calculated the energy difference between the bound exciton and its ‘free carrier’ state.
“While the former is given by the ‘optical gap’, linked to light absorption and emission, the latter is given by the ‘transport gap’, which denotes the energy needed to move an electron from the highest bound energy level to the lowest free energy level,” according to the university.
Experimental photo-luminescence and photo-absorption were used to determine the optical gap, while the transport gap was found though ultra-violet photo-electron spectroscopy and low-energy inverse photo-electron spectroscopy, “a technique pioneered by the research group” said Chiba.
Exciton binding energies were determined within 0.1eV – “The researchers believe that this precision level can help discuss the exciton nature of organic semiconductors with much higher confidence than previous studies,” added the university. “The researchers believe that these findings are likely to be included in future textbooks.”
Chiba University worked with the RIKEN Center for Emergent Matter Science and Hiroshima University.
The results are published as ‘Dependence of Exciton Binding Energy on Bandgap of Organic Semiconductors‘ in the Journal of Physical Chemistry Letters – available in full without payment.
Image credit: Hiroyuki Yoshida, Chiba University
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News