“In recent times, conductible hydrogels have attracted great attention as bioelectrode materials owing to their flexibility, compatibility, and excellent interaction ability,” according to the Institute. “However, the absence of injectability and degradability in conventional conductive hydrogels limits their convenience of use and performance in biological systems.”
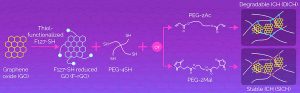
The team picked graphene oxide as a starting material, due to its large surface area, conductivity and mechanical properties.
Two different polyethylene glycols (PEG-2Mal and PEG-2Ac) were combined with this to make injectable hydrogels, resulting in two types of electrode: long-lasting (‘SICH’) and degradeable (‘DICH’) respectively.
“The researchers found that the novel injectable conductive hydrogels out-performed various existing ones by binding well to tissues and recording high signals,” said Gwangju. “Outside a living organism, SICH did not degrade for a month, while DICH showed gradual degradation from the third day onwards.”
Implanted onto mouse skin, DICH disappeared after three days, while SICH retained its shape for up to seven days.
Both were skin-compatible, it added, and both “surpassed the performance of traditional metal electrodes” when used for electromyography in rat muscle and skin. SICH worked for up to three weeks and DICH signals were completely lost after five days.
The study is published in the Small journal as ‘Injectable conductive hydrogels with tunable degradability as novel implantable bioelectrodes‘ – payment required for full access.
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News