The multi-step process used to search for possible electrolytes reduced a list of 32 million inorganic materials to 18 in 80 hours, using ~1,000 virtual machines in Microsoft’s Azure Quantum Elements cloud.
“We think there’s an opportunity to do this across a number of scientific fields,” said PNNL chief digital officer Brian Abrahamson.
Several artificial intelligence algorithms were trained for the demonstration.
The first evaluated elements for suitability, and tried ions of these in known crystal structures – producing the 32 million starting compounds.
The next eliminated unstable materials, then another filtered out candidate molecules based on reactivity, and another based on their potential to conduct ions.
“The idea isn’t to find every single possible needle in the hypothetical haystack, but to find most of the good ones,” according to Microsoft. “AI technology whittled the 32 million down to about 500,000 mostly new stable materials, then down to 800.”
Theoretical modelling was used next – more accurate that the AI estimates, but requiring far more computing power than AI, and therefore taking more time.
The first high-performance computing (HPC) step used density functional theory to calculate the energy of each material relative to all the other states it could be in, said Microsoft, then molecular dynamics simulations combining AI and HPC analysed the movements of ions inside each material.
At this point 150 chemicals remained standing, whittled by HPC using cost, availability and other practical metrics down to 23.
In total, 90% of computing time was spent on AI algorithms and the rest on mathematical simulation.
Five of 23 these were already known, leaving 18, from which PNNL materials scientists selected six to be made and tested in batteries.
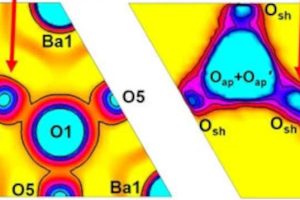
Already, one group of materials (top photo) has turned out to be a conductor of both lithium and sodium ions. They have particular structures and are related by the formula NaxLi3-xYCl6, where x is between 0 and three. Na2LiYCl6 was particularly successful.
“It was thought that sodium ions and lithium ions couldn’t be used together in a solid-state electrolyte system because they are similarly charged but have different sizes,” said Microsoft. “It was assumed that the structural framework of a solid-state electrolyte material couldn’t support the movement of two different ions.”
 But after testing, “we found that the sodium and lithium ions seem to help each other”, said PNNL materials scientist Vijay Murugesan (left).
But after testing, “we found that the sodium and lithium ions seem to help each other”, said PNNL materials scientist Vijay Murugesan (left).
Others likely electrolytes from the sifting process have the lithium, yttrium and chlorine, but not the sodium – meaning that all of the ‘winners’ can be made from the same three or four elements, even though 54 elements were originally fed in to the process.
Even if none of the materials ever make it into batteries “the speed at which we found a workable battery chemistry is pretty compelling”, said Brian Abrahamson, responsible for increasing the use of digital technology at PNNL.
 Electronics Weekly Electronics Design & Components Tech News
Electronics Weekly Electronics Design & Components Tech News